- Initial patient compliance with fecal immunochemical testing (FIT) was high at 48.2%.
- Follow-up screening remained high at 75.3% to 86.1% for 4 years.
- Previous studies have found FIT to be sensitive for occult blood in stool, as well as for polyps.
- FIT has higher compliance and similar sensitivity as fecal occult blood testing (FOBT).
- Positive result on FIT screening should prompt the more invasive CT colonoscopy or the fully invasive colonoscopy for conclusive results.
A number of recent studies show that low-cost fecal immunochemical testing (FIT) (Figure 1) is an excellent routine, at-home early screening modality for early detection of potential colon polyps or colon cancer. Positive FIT results should prompt clinicians to move to still more sensitive tests such as full cathartic preparation CT colonography (f-CTC) or optical colonoscopy (OC) for conclusive results.
FIT is Accurate, Cost-Effective, Provides High Compliance Rates
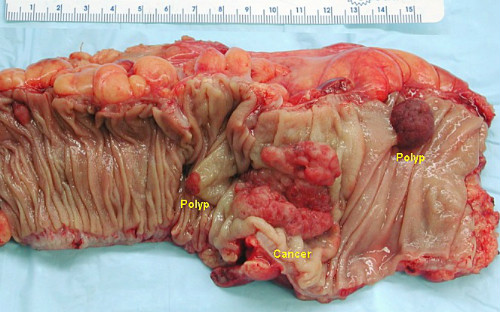
A retrospective cohort study published recently in The Annals of Internal Medicine show that the fecal immunochemical test (FIT) is convenient for patients and sensitive for detecting early precursors to colorectal cancer (CRC) (Figure 2).
The study reviewed the records of 323,349 Kaiser Permanente health plan members in Northern and Southern California aged 50 to 70 years. The subjects began FIT testing in 2007 or 2008, completed the first round of FIT, and were available for follow-up for up to 4 subsequent screening rounds.
The review showed evaluated screening participation, FIT positivity (≥20 µg of hemoglobin/g), positive predictive values for adenoma and CRC, and FIT sensitivity for detecting CRC obtained from Kaiser Permanente electronic databases and cancer registries.
Of the patients included in the screening arm, 48.2% participated in round 1. Of those who remained eligible, 75.3% to 86.1% participated in subsequent rounds. Median follow-up was 4.0 years, and 32% of round 1 participants crossed over to endoscopy over 4 screening rounds—7.0% due to a positive FIT result.
The FIT positivity rate (5.0%) and positive predictive values (adenoma, 51.5%; CRC, 3.4%) were highest in round 1. Overall, FIT screening detected 80.4% of patients with CRC diagnosed within 1 year of testing, including 84.5% in round 1 and 73.4% to 78.0% in subsequent rounds.
“Annual FIT screening was associated with high sensitivity for CRC, with high adherence to annual follow-up screening among initial participants. The findings indicate that annual programmatic FIT screening is feasible and effective for population-level CRC screening,” the reviewers concluded.
Evidence for FIT Sensitivity
A study published recently in the Journal of the National Cancer Institute reported a randomized clinical trial comparing reduced-preparation CT colonography (r-CTC), full-preparation CT colonography (f-CTC), FIT, and OC (Figure 3) for population screening for CRC. The study included 16,087 subjects from a district of Florence, Italy, aged 54 to 65 years.
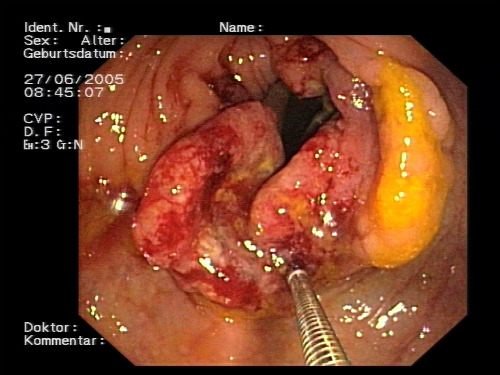
Subjects were randomly assigned to be invited by mail to 1 of 4 screening interventions: 1) biennial FIT for 3 rounds, 2) r-CTC, 3) f-CTC, or 4) OC. Patients tested positive to FIT or CTC (≤1 polyp ≥6mm) were referred to OC work-up. The primary outcomes were participation rate and detection rate (DR) for cancer or advanced adenoma (advanced neoplasia).
Participation rates were 50.4% (4677/9288) for first-round FIT, 28.1% (674/2395) for r-CTC, 25.2% (612/2430) for f-CTC, and 14.8% (153/1036) for OC. All differences between groups were statistically significant (P=0.047 for r-CTC vs f-CTC; P<0.001 for all others).
Detection rates for advanced neoplasia were 1.7% (79/4677) for first-round FIT, 5.5% (37/674) for r-CTC, 4.9% (30/612) for f-CTC, and 7.2% (11/153) for OC. Differences in DR between CTC groups and FIT were statistically significant (P<0.001), but not between r-CTC and f-CTC (P=).65).
“Reduced preparation increases participation in CTC. Lower attendance and higher DR of CTC as compared with FIT are key factors for the optimization of its role in population screening of CRC,” the authors concluded.
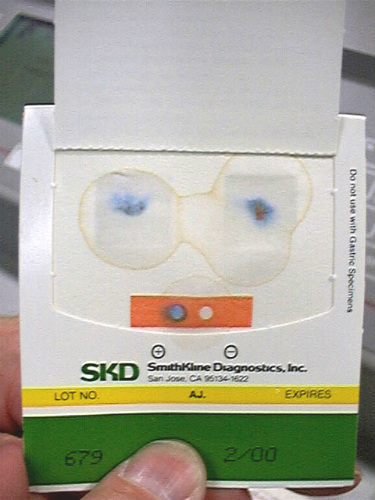
Evidence for FIT Compliance
An earlier study published in Best Practice & Research in Clinical Gastroenterology reported on an Asian population where nearly 45% of all worldwide CRC cases occur. Due to the high rate of CRC in Asia, the need for an easy, cost-effective screening modality with high compliance is especially urgent. Stool-based tests, including guaiac fecal occult blood test (gFOBT) (Figure 4) and fecal immunochemical test (FIT), can select subjects at risk of significant colorectal neoplasms from the large target population. For this reason, these are currently the most commonly used non-invasive screening tools. FIT has been shown to have higher sensitivity than gFOBT for early neoplasms.
FIT also offers the ability to provide high-throughput automatic analysis, and increased compliance among the public has the potential to offer greater effectiveness for reducing CRC incidence and mortality. Owing to the large target population and constrained endoscopic capacity and manpower, FIT is nowadays the most popular CRC screening test in Asia.
