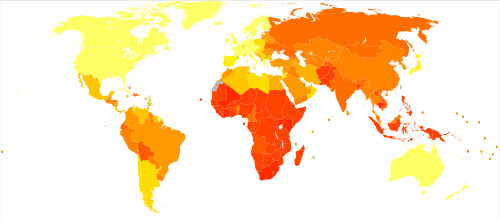
- The U.S. death rate from tuberculosis (TB) has increased recently.
- TB remains highly contagious and frequently lethal.
- A number of U.S. states have seen increases in TB rates and mortality.
- An accurate TB detection assay could considerably reduce healthcare expenditures.
Nearly 9,500 cases of TB were reported in the U.S. in 2014, according to the Centers for Disease Control and Prevention website. This represents a rate of approximately 3 cases per 100,000 persons. In year 2013, 555 people died of complications related to TB in the U.S. Both the number of TB cases reported and the case rate decreased; this represents a 1.5% and 2.2% decline, respectively, compared to 2013. This is the smallest decline in more than a decade, according to the CDC website. (Figure 1.)
“Rapid and accurate case detection is critical for effective treatment, prevention of transmission of infection, treatment failures, relapse, and development of resistant cases. As we are lagging behind the targets of STOP TB, consistent research and funding is required for the development of propagation of the best monitoring centers,” the authors explained. The “Xpert MTB/RIF assay is a robust, sensitive, and specific test for accurate diagnosis of tuberculosis (Figure 2) as compared to conventional tests like culture and microscopic examination,” they concluded. The test was found to be particularly helpful in patients with HIV.
The Analysis
A systematic literature search was conducted of the Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews, MEDLINE, PUBMED, Scopus, Science Direct, and Google Scholar for relevant studies published between 2010 and December 2014. Studies given in the systematic reviews were accessed separately and used for analysis. Included studies evaluated the diagnostic accuracy of Xpert MTB/RIF assay among adult or predominantly adult patients (≥14 years) who were presumed to have pulmonary TB, with or without HIV infection. Also, studies that had assessed the diagnostic accuracy of Xpert MTB/RIF assay using sputum and other respiratory specimens were included (Figure 4).
Results
“The included studies had a low risk of any form of bias, showing that findings are of high scientific validity and credibility” the authors noted. This analysis shows that Xpert MTB/RIF is an accurate diagnostic test for TB and detection of rifampicin resistance.
According to the WHO data for 2011, only 48% of the MDR-TB patients detected were treated successfully. Of these patients, 16% died and 12% were not cured regardless of treatment. Desired outcomes were not achieved in the remainder of patients. The treatment success rate was only 22% for XDR TB. “WHO has set high goals to end the global TB epidemic such that there should be a 95% reduction in TB deaths and a 90% decrease in TB incidence by year 2035 as compared to the 2015 targets,” the authors added.
