Hydroxyurea Interferes with Continuous Glucose Monitoring System Readings in Patient With Diabetes
By Andrew John, /alert Contributor
June 8, 2021
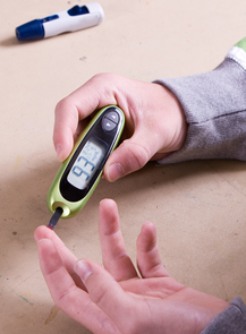
The drug hydroxyurea interfered with continuous glucose monitoring in a woman with type 1 diabetes, according to a clinical observation letter published in Diabetes Care.
“While currently available continuous glucose monitoring (CGM) systems have continually improving accuracy that has revolutionized modern diabetes care, potential medication interferences are recognized for all available CGM systems,” Emily D. Szmuilowicz, MD, and Grazia Aleppo, MD, of the Northwestern University Feinberg School of Medicine, Chicago, wrote. “For Dexcom CGM systems, hydroxyurea was recently recognized as an interfering substance that can falsely elevate sensor glucose readings, but the data demonstrating this interference have not been previously published.”

A 69-year-old woman with type 1 diabetes took 1,000 mg of hydroxyurea daily for essential thrombocytosis, the authors wrote. The woman used both an insulin pump and a real-time glucose monitoring system.
Every time the woman took her daily dose of hydroxyurea, she reported to clinicians, her sensor glucose readings would increase for the next 6 hours. This occurred even while she was fasting, and happened regardless of what time she took the drug. Further, Szmuilowicz and Aleppo wrote, the glucose management indicator based on the 14-day average of sensor glucose readings was higher than that of the patient’s glycated hemoglobin, which suggested “artifactual discordance.”
Upon approval from the Northwestern University Institutional Review Board, the authors instructed the patient to wear two different continuous monitoring systems — the Dexcom G6 (San Diego, California) and the FreeStyle Libre 2 (Abbott, Abbott Park, Illinois) — for 14 days. This period included three test days during which the patient fasted for 4 hours before taking hydroxyurea and for a minimum of 8 hours after.
Both systems took glucose readings that were similar to levels determined in laboratory plasma tests, the authors reported. They also demonstrated parallel rises following mealtimes. However, after the woman took hydroxyurea while fasting, the two systems showed different results: the Dexcom system showed increased glucose readings for about 6 to 10 hours following the administration of hydroxyurea, whereas the Libre system did not.
The maximum sensor glucose readings following hydroxyurea taken in the fasting state on each of the 3 test days were:
8 November, 10 p.m.: Dexcom 254 mg/dL (Libre 128 mg/dL)
10 November, 11:50 p.m.: Dexcom 216 mg/dL (Libre 90 mg/dL)
11 November, 10 p.m.: Dexcom 194 mg/dL (Libre 89 mg/dL)
“In summary, our case demonstrated for the first time that hydroxyurea is an interferent in a commonly used CGM system, and providers must be aware that these CGM data must be disregarded for several hours after each hydroxyurea administration,” Szmuilowicz and Aleppo wrote. “This medication-induced interference with CGM and by extension hybrid closed-loop insulin delivery is fortunately rare, and these minor limitations pale in comparison with the wide-ranging, unprecedented, and revolutionary benefits that these technologies have afforded to people living with diabetes.”
Aleppo and Szmuilowicz wrote that the incident offered three lessons: that clinicians should listen to patients even when patients report phenomena that have not been corroborated by medical literature; that the treatment modifications made in response to this woman’s case were based on information that might have been “lost” if the authors relied solely on web-based, remotely obtained electronic information; and that “in embracing the revolutionary and ever-growing benefits of CGM use, we must also pause to consider the infrequent instances of limitation, no matter how rare.”
Disclosures: Aleppo reports consulting roles with Dexcom, Inc., and Insulet Corporation, and has research support from Dexcom, Eli Lilly, and Insulet Corporation. Szmuilowicz reports no relevant financial disclosures.








.jpg)