Novo Nordisk Recalls Insulin Samples Citing Storage Concerns
By Adam Hochron
May 14, 2021
Novo Nordisk has issued a voluntary recall for several product samples due to issues with storage temperatures, according to a release from the manufacturer.
The recall applies to samples for the diabetes medications insulin detemir (Levemir), insulin degludec (Tresiba), insulin aspart (Fiasp and Novolog), and insulin degludec and liraglutide injection (Xultophy).
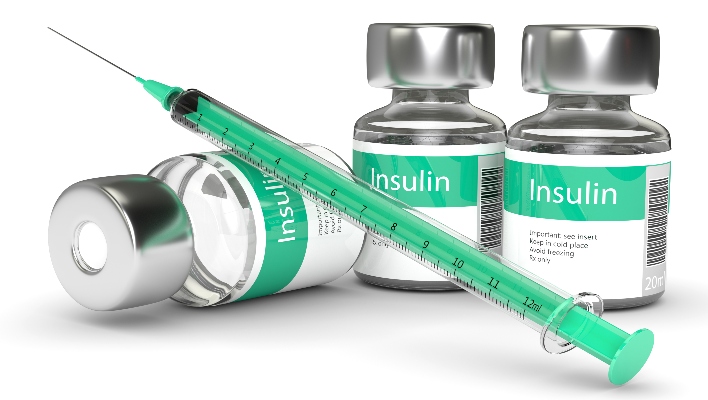
The recall was announced after it was determined the samples might have been stored at temperatures below the required levels. According to the release, the medications could lose their full efficacy or be damaged at temperatures below 32°F. A damaged cartridge or injector could result in patients not receiving the proper dose. As a result, patients could be at risk of developing hyperglycemia or hypoglycemia and suffering potentially life-threatening health consequences.
The full list of recalled products can be found here.
The affected medications can be identified based on their batch or lot number found on the product or carton. Doctors who received the affected samples have been contacted by the manufacturer and asked to return the impacted samples. Any patients who have been given one of the affected samples should have received a letter from their provider notifying them of the recall.
The FDA was notified about the recall, which does not apply to products that have been distributed to pharmacies or mail-order services.








.jpg)