FDA Approves Semglee, First-ever Insulin Biosimilar, For Use in Diabetes
By Andrew John, /alert Contributor
August 10, 2021
The FDA has approved semglee, an interchangeable biosimilar insulin product, for use in diabetes, the agency has announced. Semglee, produced by Mylan Pharmaceuticals, is the first biosimilar insulin product to gain FDA approval.
“This is a momentous day for people who rely daily on insulin for treatment of diabetes, as biosimilar and interchangeable biosimilar products have the potential to greatly reduce health care costs,” Janet Woodcock, MD, the acting FDA commissioner, said in a press release. “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.”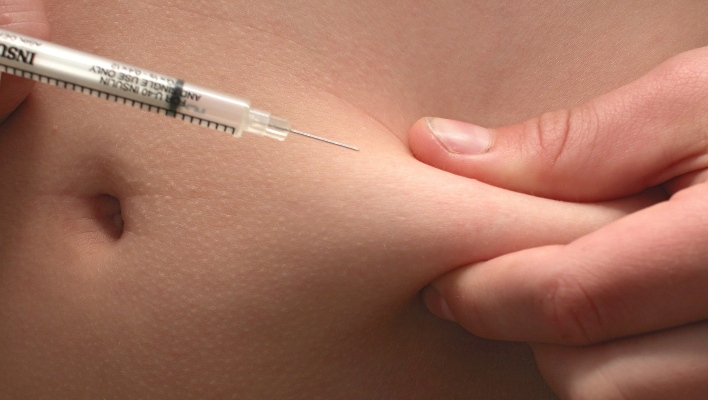
Semglee (insulin glargine-yfgn) is a long-acting synthetic insulin product that can be used as a substitute for lantus (insulin glargine), with which it has “no meaningful clinical differences,” in both type 1 and type 2 diabetes, the FDA announcement said.
Over 34 million Americans have diabetes. Biosimilar products, which can be substituted for so-called “reference products” at lower prices than the original without any input from the prescribing physician, can translate into lowered health care costs for many patients. The FDA said biosimilar products generally launch with list prices between 15% and 35% cheaper than the products they are designed to replace.
“Access to affordable insulin is critical and long-acting insulin products, like insulin glargine, play an important role in the treatment of Types 1 and 2 diabetes mellitus,” Peter Stein, MD, director of the Office of New Drugs in the FDA’s Center for Drug Evaluation and Research, said in the announcement. “The FDA’s high standards for approval mean health care professionals and patients can be confident in the safety and effectiveness of an interchangeable biosimilar product, just as they would for the reference product.”
Semglee is injected subcutaneously and sold in 10 mL vials and 3 mL prefilled pens. Serious side effects of semglee include hypokalemia, hypoglycemia, heart failure and severe allergic reactions. It is not recommended for diabetic ketoacidosis, the press release said, and should not be used during hypoglycemic episodes.








.jpg)