The U.S. Food and Drug Administration has released a warning reminding patients that thermography should not replace mammography for breast cancer screening.
According to the FDA, the company has received reports from patients and providers saying that some health care centers and websites are providing information that misleads them to believe that thermography, an imaging test that shows heat and blood flow on or near the surface of the body, is a “proven alternative to mammography”.
.jpg)
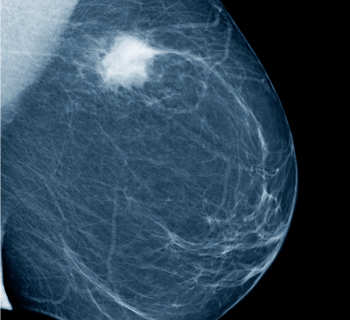
Figure 1. Thermography of a breast.
Figure 2. A mammogram showing a cancerous mass in white. (Source: FDA)
“Plenty of evidence shows that mammography is still the most effective screening method for detecting breast cancer in its early, most treatable stages,” Helen J Barr, MD, director of the Division of Mammography Quality Standards in the FDA’s Center for Devices and Radiological Health, said in a press release. “You should not rely soley on mammography for the screening or diagnosis of breast cancer.”
The FDA has only cleared the use of thermography in conjunction with mammography, according to the website. The company states in their report that patients should not undergo a thermography test alone as there is no current evidence that the test accurately detects breast cancer.
“Some websites claim that thermography can find breast cancer years before it would be detected through other methods and have unproven claims about improved detection of cancer in dense breasts,” the company said. “The FDA is aware of no evidence that supports these claims.”
In 2016, the FDA issued two warnings to companies that provided false advertisement and information on thermography. One warning letter was issued to Thermogram Assessment Services, a thermography firm based in California, for marketing its thermographic software without marketing clearance or approval.
The second warning letter was issued to Nature’s Treasures, Inc., a website that is currently “under maintenance”, for marketing their telethermographic system without clearance and claiming that “thermography can detect the possibility of breast cancer” much earlier than mammography, among other statements.
The FDA stated that it will continue to monitor the situation. For more information, visit https://www.fda.gov/.
