- Dabigatran anti-coagulation can now be reversed.
- FDA approves idarucizumab for reversal of dabigatran anti-coagulation.
- Normal coagulation is established “in minutes.”
- Preliminary data show idarucizumab to be safe and effective for reversal of dabigatran anti-coagulation.
- Both efficacy and safety trials are sponsored by the manufacturer and published in top-tier journals.
For the first time since its introduction into the market the potent anticoagulant dabigatran has a reversal agent. Idarucizumab was recently approved based largely on data published in the New England Journal of Medicine.
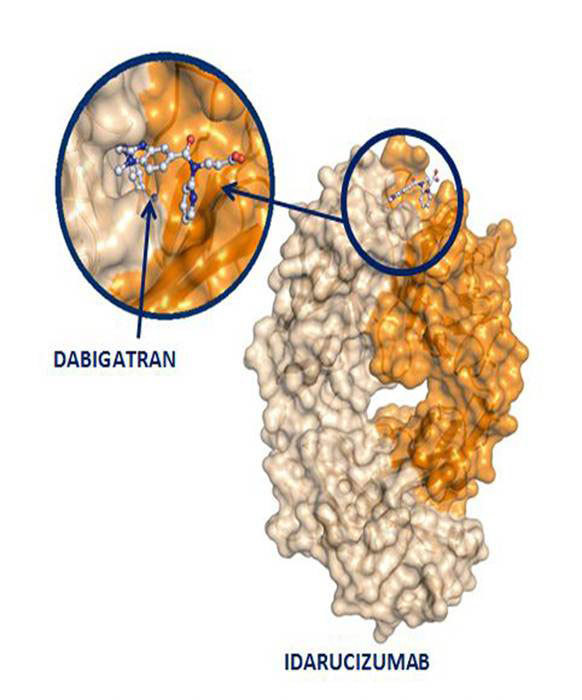
(Image courtesy of Boehringer Ingelheim.)
Efficacy
The trial, of which interim findings were published, is being funded by the manufacturer of idarucizumab, Boehringer Ingelheim. The results in 91 patients have thus far shown that idarucizumab is a potent and effective reversal agent for dabigatran. Coagulation was said to have been re-established within minutes, the researchers reported.
“This interim analysis included 90 patients who received idarucizumab (51 patients in group A and 39 in group B). Among 68 patients with an elevated dilute thrombin time and 81 with an elevated ecarin clotting time at baseline, the median maximum percentage reversal was 100% (95% confidence interval, 100 to 100). Idarucizumab normalized the test results in 88 to 98% of the patients, an effect that was evident within minutes. Concentrations of unbound dabigatran remained below 20 ng per milliliter at 24 hours in 79% of the patients. Among 35 patients in group A who could be assessed, hemostasis, as determined by local investigators, was restored at a median of 11.4 hours. Among 36 patients in group B who underwent a procedure, normal intraoperative hemostasis was reported in 33, and mildly or moderately abnormal hemostasis was reported in 2 patients and 1 patient, respectively. One thrombotic event occurred within 72 hours after idarucizumab administration in a patient in whom anticoagulants had not been reinitiated,” the researchers wrote (N Engl J Med. 2015;373:511-20).
Safety
The trial NEJM study did not examine safety. However, a trial published in the Lancet did. “These phase 1 results show that idarucizumab was associated with immediate, complete, and sustained reversal of dabigatran-induced anticoagulation in healthy men, and was well tolerated with no unexpected or clinically relevant safety concerns, supporting further testing. Further clinical studies are in progress.”
The safety trial, also sponsored by Boehringer Ingelheim, included just 47 men (Lancet. 201515;386:680-90).
