- By taking tenofovir and emtricitabine daily, individuals at high risk of contracting HIV can reduce infection risk to near zero.
- When used with condoms and other prevention methods, HIV infection risk can be virtually eliminated.
- Pre-exposure prophylaxis combines drug therapy, physical barriers, and immune-system conditioning to reduce the risk of HIV infection during exposure to the virus.
- Numerous important studies have demonstrated the potential for this strategy to reduce HIV-infection rates.
Pre-exposure prophylaxis, or PrEP, has been shown to greatly reduce the risk of HIV infection (Figure 1) in people who are at substantial risk of infection. By taking a combination of tenofovir and emtricitabine (Truvada, Gilead; Figure 2) daily, a person who is exposed to HIV through sex or injection drug use will experience significantly reduced risk of acquiring the infection. The drugs appear to prevent the virus from establishing a permanent infection, according to a large body of published research.
When taken consistently, PrEP has been shown to reduce the rate of HIV infection by as much as 92% in people who are at high risk. The regimen, however, is much less effective when it is not taken consistently.
“PrEP is a powerful HIV prevention tool and can be combined with condoms and other prevention methods to provide even greater protection than when used alone. But people who use PrEP must commit to taking the drug every day and seeing their health care provider for follow-up every 3 months,” according to the website of the Centers for Disease Control and Prevention (CDC).
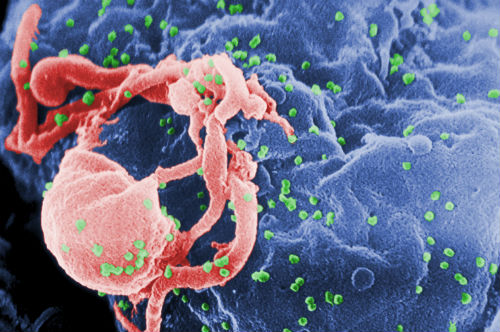
budding from a cultured lymphocyte.
(Sources: Wikipedia/By C. Goldsmith Content Providers: CDC/ C. Goldsmith, P. Feorino,
E. L. Palmer, W. R. McManus; The Centers for Disease Control and Prevention's
Public Health Image Library/Public Domain.)
JAMA Internal Medicine. 2016
“The incidence of HIV acquisition was extremely low despite a high incidence of STIs in a large US PrEP demonstration project. Adherence was higher among those participants who reported more risk behaviors. Interventions that address racial and geographic disparities and housing instability may increase the impact of PrEP,” concluded the authors of a study published recently in JAMA Internal Medicine.
A number of randomized, clinical trials have demonstrated the effectiveness of PrEP in preventing HIV infection. This study examined PrEP adherence, sexual behaviors, and the incidence of STIs and HIV infection in a cohort of MSM and transgender women initiating PrEP in the U.S.
The study was initiated on October 1, 2012 and continued through February 10, 2015. The trial include 557 MSM and transgender women in 2 STI clinics in San Francisco, California, and Miami, Florida, and a community health center in Washington, DC.
The interventions included a combination of daily, oral tenofovir/emtricitabine which was provided free of charge for 48 weeks. All participants received HIV testing, brief client-centered counseling, and clinical monitoring.
Adherence to the regimen was evaluated through blood sampling, self-reported numbers of anal sex partners, episodes of condomless receptive anal sex, and incidence of STI and HIV acquisition.
“Overall, 557 participants initiated PrEP, and 437 of these (78.5%) were retained through 48 weeks. Based on the findings from the 294 participants who underwent measurement of tenofovir diphosphate levels, 80.0% to 85.6% had protective levels (consistent with ≥4 doses/wk) at follow-up visits.
“African American participants (56.8% of visits; P=0.003) and those from the Miami site (65.1% of visits; P<0.001) were less likely to have protective levels, whereas those with stable housing (86.8%; P=0.02) and those reporting at least 2 condomless anal sex partners in the past 3 months (88.6%; P=0.01) were more likely to have protective levels.
“The mean number of anal sex partners declined during follow-up from 10.9 to 9.3, whereas the proportion engaging in condomless receptive anal sex remained stable at 65.5% to 65.6%. Overall STI incidence was high (90 per 100 person-years) but did not increase over time. Two individuals became HIV infected during follow-up (HIV incidence, 0.43 [95% CI, 0.05-1.54] infections per 100 person-years); both had tenofovir diphosphate levels consistent with fewer than 2 doses/wk at seroconversion,” the authors explained.

(Sources: Wikipedia/By Jeffrey Beall - Own work/Creative Commons.)
Mortality and Morbidity Weekly Report, 2015
“A substantial number of MSM, persons who inject drugs, and heterosexually active adults have indications for PrEP. Efforts to increase knowledge of and access to PrEP should accompany efforts to increase early diagnosis and treatment of persons with HIV infection to achieve the prevention benefits of viral suppression. Reducing disparities in access to clinical care for the prevention and treatment of HIV infection can accelerate achieving the National HIV/AIDS Strategy 2020 goal for reducing the number of new HIV infections in the United States,” according to an article published in the CDC’s Mortality and Morbidity Weekly Report (MMWR) in late 2015.
Approximately 40,000 people in the U.S. are diagnosed annually with human immunodeficiency virus (HIV; FIgure 3) infection. PrEP with daily oral antiretroviral medication is a relatively new, highly effective intervention that has been shown to reduce the number of new HIV infections, according to the CDC.
“Approximately 24.7% of sexually active adult men who have sex with men (MSM) (492,000 [95% confidence interval {CI}=212,000–772,000]), 18.5% of persons who inject drugs (115,000 [CI=45,000–185,000]), and 0.4% of heterosexually active adults (624,000 [CI=404,000–846,000]), had substantial risks for acquiring HIV consistent with PrEP indications,” the CDC authors wrote in MMWR.
“Based on current guidelines,” the authors continued, “many MSM, persons who inject drugs, and heterosexually active adults have indications for PrEP. A higher percentage of MSM and persons who inject drugs have indications for PrEP than heterosexually active adults, consistent with distribution of new HIV diagnoses across these populations.”
The CDC has called for public health practitioners, clinical organizations, health departments, and community-based organizations to work to increase awareness of the efficacy and availability of PrEP for people at risk of contracting HIV. “These data can be used to inform scale-up and evaluation of PrEP coverage. Increasing delivery of PrEP and other highly effective HIV prevention services could lower the number of new HIV infections occurring in the United States each year,” the authors explained.
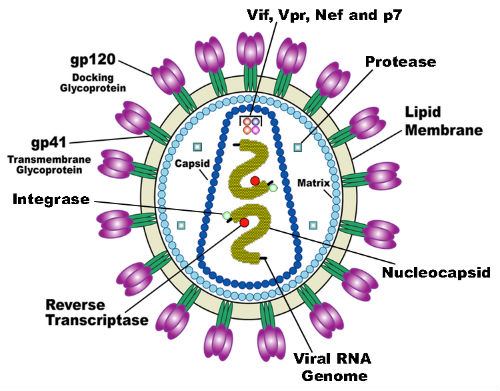
(Sources: Wikipedia Commons/By U.S. National Institute of Health
(Redrawn by en: User: Carl Henderson)/Public Domain.)
The Lancet, 2013
“In this study, daily oral tenofovir reduced the risk of HIV infection in people who inject drugs. Pre-exposure prophylaxis with tenofovir can now be considered for use as part of an HIV prevention package for people who inject drugs,” concluded the authors of this study published in The Lancet.
As has been demonstrated in a number of well-constructed randomized, controlled trials and systematic reviews, antiretroviral PrEP reduces rates of HIV infection. This study was designed to assess whether daily oral use of tenofovir could reduce HIV infection among injection drug users.
The randomized, double-blind, placebo-controlled trial, enrolled volunteers from 17 drug-treatment clinics in Bangkok, Thailand. Eligible participants included those aged 20-60 years who were HIV-negative, and reported injecting drugs during the previous year.
“We randomly assigned participants (1:1; blocks of 4) to either tenofovir or placebo using a computer-generated randomization sequence. Participants chose either daily directly observed treatment or monthly visits and could switch at monthly visits. Participants received monthly HIV testing and individualized risk-reduction and adherence counselling, blood safety assessments every 3 months, and were offered condoms and methadone treatment. The primary efficacy endpoint was HIV infection, analyzed by modified intention-to-treat analysis,” the authors explained.
“Between June 9, 2005, and July 22, 2010, we enrolled 2413 participants, assigning 1204 to tenofovir and 1209 to placebo. Two participants had HIV at enrolment and 50 became infected during follow-up: 17 in the tenofovir group (an incidence of 0·35 per 100 person-years) and 33 in the placebo group (0·68 per 100 person-years), indicating a 48·9% reduction in HIV incidence (95% CI 9·6-72·2; P=0·01). The occurrence of serious adverse events was much the same between the two groups (P=0·35). Nausea was more common in participants in the tenofovir group than in the placebo group (p=0·002),” the authors noted
The New England Journal of Medicine, 2012
Once-daily oral tenofovir, combination tenofovir–emtricitabine were both found to protect against HIV-1 infection in heterosexual men and women,” concluded the authors of a randomized trial published in The New England Journal of Medicine.
“We conducted a randomized trial of oral antiretroviral therapy for use as preexposure prophylaxis among HIV-1–serodiscordant heterosexual couples from Kenya and Uganda. The HIV-1–seronegative partner in each couple was randomly assigned to 1 of 3 study regimens: once-daily tenofovir; combination tenofovir–emtricitabine; or matching placebo; and followed monthly for up to 36 months. At enrollment, the HIV-1–seropositive partners were not eligible for antiretroviral therapy, according to national guidelines. All couples received standard HIV-1 treatment and prevention services.”
“We enrolled 4758 couples, of whom 4747 were followed: 1584 randomly assigned to tenofovir, 1579 to tenofovir–emtricitabine, and 1584 to placebo. For 62% of the couples followed, the HIV-1–seronegative partner was male.
“Among HIV-1–seropositive participants, the median CD4 count was 495 cells per cubic millimeter (interquartile range, 375 to 662). A total of 82 HIV-1 infections occurred in seronegative participants during the study, 17 in the TDF group (incidence, 0.65 per 100 person-years), 13 in the TDF–FTC group (incidence, 0.50 per 100 person-years), and 52 in the placebo group (incidence, 1.99 per 100 person-years), indicating a relative reduction of 67% in the incidence of HIV-1 with TDF (95% confidence interval [CI], 44 to 81; P<0.001) and of 75% with TDF–FTC (95% CI, 55 to 87; P<0.001).
“Protective effects of tenofovir–emtricitabine and tenofovir alone against HIV-1 were not significantly different (P=0.23), and both study medications significantly reduced the HIV-1 incidence among both men and women.
“The rate of serious adverse events was similar across the study groups. Eight participants receiving active treatment were found to have been infected with HIV-1 at baseline, and among these eight, antiretroviral resistance developed in two during the study,” the authors explained.
The New England Journal of Medicine, 2010
“Oral emtricitabine and tenofovir provided protection against the acquisition of HIV infection among the subjects. Detectable blood levels strongly correlated with the prophylactic effect,” concluded the authors of this study published in The New England Journal of Medicine.
The researchers conducted a randomized, placebo-controlled trial that included 2499 HIV-seronegative men or transgender women who have sex with men. Participants were randomly assigned to receive emtricitabine and tenofovir, or placebo once daily. All subjects received HIV testing, risk-reduction counseling, condoms, and management of sexually transmitted infections.
“The study subjects were followed for 3324 person-years (median, 1.2 years; maximum, 2.8 years). Of these subjects, 10 were found to have been infected with HIV at enrollment, and 100 became infected during follow-up (36 in the FTC–TDF group and 64 in the placebo group), indicating a 44% reduction in the incidence of HIV (95% confidence interval, 15 to 63; P=0.005).
“In the emtricitabine and tenofovir group, the study drug was detected in 22 of 43 of seronegative subjects (51%) and in 3 of 34 HIV-infected subjects (9%) (P<0.001). Nausea was reported more frequently during the first 4 weeks in the emtricitabine and tenofovir group than in the placebo group (P<0.001). The two groups had similar rates of serious adverse events (P=0.57),” the authors explained.
