- Would extending aromatase inhibitor (AI) treatment to 10 years would further reduce the risk of breast cancer recurrence?
- Prolonged AI therapy avoid adverse effects of treatment with tamoxifen.
- Trial enrolled 1,918 women with early stage breast cancer.
- Extending AI treatment to 10 years significantly improved disease-free survival.
- Results were presented at 2016 annual meeting of the American Society of Clinical Oncology; not yet published in peer-reviewed journal.
Extending aromatase inhibitor (AI) therapy to 10 years significantly improves disease-free survival in postmenopausal women who have survived early-stage breast cancer. Compared to 5 years of AI treatment as initial therapy or preceded by 2-5 years of tamoxifen, extending AI treatment to 10 years significantly improved disease-free survival (DFS) and overall survival (OS). The findings were presented at the 2016 annual meeting of the American Society of Clinical Oncology (ASCO). The data have recently been published in the New England Journal of Medicine.
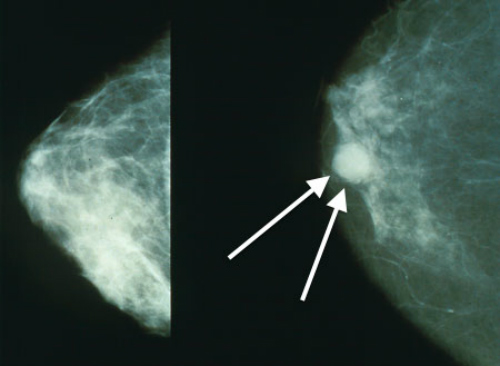
and a breast with cancer (right, white arrows).
(Sources: Wikipedia/Bakerstmd/Creative Commons.)
The researchers conducted a multicenter, randomized trial to examine the clinical differences of extending adjuvant letrozole, an aromatase inhibitor, for 5 years after patients completed an initial 5 years of AI therapy alone or preceded by tamoxifen.
Those who received Femara [letrozole] for the 5 additional years had a 34% lower risk of breast cancer recurrence than those who received the placebo, said lead researcher Paul Goss, MD, Professor of Medicine at Harvard Medical School and Chief of Breast Cancer Research at Massachusetts General Hospital.
Five years of AI therapy either as primary treatment or following 2-5 years of tamoxifen is the current standard of care for postmenopausal women with hormone receptor positive early breast cancer. These researchers examined the question of whether extending AI treatment to 10 years would further reduce the risk of breast cancer recurrence.
The use of an AI instead of tamoxifen may also enable more women to adhere to treatment guidelines. The side effects of tamoxifen are deemed unpleasant by many women and this may be a reason that these women discontinue treatment prematurely in the followup period.
"Aromatase inhibitors do not have all the side effects of tamoxifen and overall are much better tolerated than tamoxifen," explained Stephanie Bernik, MD, in an interview with HealthDay that was published in U.S. News. Dr. Bernik is Chief of Surgical Oncology at Lenox Hill Hospital, part of the North Shore-LIJ Health System, in New York City.
(Sources: Wikipedia/Creative Commons/Lokal_Profil CC BY-SA 2.5.)
The Analysis
This double-blind, placebo-controlled trial tested examined the effects extending AI treatment with letrozole for an additional 5 years. The primary endpoint of the trial was DFS. The trial enrolled 1,918 women with early stage breast cancer were enrolled. The median follow-up was 6.3 years. The researchers recorded 165 DFS events occurred—67 among patient taking letrozole and 98 among those receiving placebo. Of these events, 42 were distant metastases among women taking letrozole and 53 were among women who received placebo. There were 200 deaths in all (100 in each treatment group).
The 5-year DFS was 95% among patients receiving letrozole versus 91% for those who received placebo (HR 0.66; P=0.01) based on results of a 2-sided log-rank test. The log-rank test was stratified by nodal status, prior adjuvant chemotherapy, interval between last dose of AI therapy and randomization, and duration of prior tamoxifen treatment at randomization. The 5-year overall survival (OS) rate was 93% for patients taking letrozole and 94% for those taking a placebo with a hazard ratio (HR) of 0.97 (P=0.83). The annual incidence rate of contralateral breast cancer was 0.21% for subjects on letrozole versus 0.49% on placebo (P=0.007).
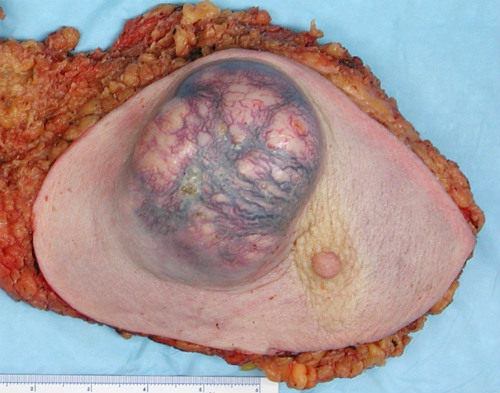
(in this case, an invasive ductal carcinoma).
(Sources: Wikipedia/ Emmanuelm/Creative Commons.)
Conclusions
“Compared to 5 years of AI treatment as initial therapy or preceded by 2-5 years of tamoxifen, extending AI treatment to 10 years significantly improves disease-free survival. Further analyses will provide a comprehensive picture of toxicities and QOL,” the authors wrote.
