- The Ex-Press mini glaucoma shunt has been available internationally for nearly 20 years with nearly 100,000 implantations worldwide.
- Safety and efficacy have become well established
- Clinical outcomes appear to be significantly superior to trabeculectomy.
- Relatively high cost of the Ex-Press shunt may be an important barrier to more widespread implementation.
- The device is referred to as a “glaucoma filtration device” by the manufacturer.
Numerous well-conducted studies, meta-analyses, and systematic reviews support the safety and efficacy of the Ex-Press shunt (Figure 1) for the treatment of uncontrolled glaucoma. The device has been implanted in nearly 100,000 patients (Figure 2) and has proven to be significantly more effective than trabeculectomy. (Figure 3.)
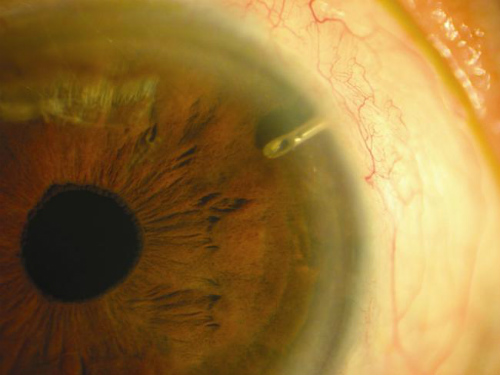
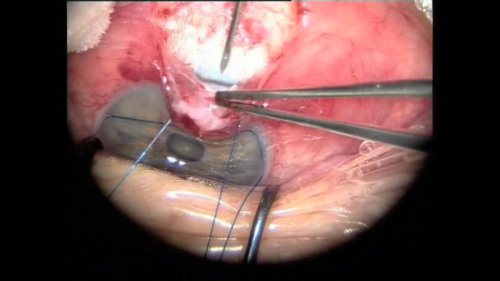
“Glaucoma drainage devices are designed to divert aqueous humor from the anterior chamber to an external reservoir, where a fibrous capsule forms about 4-6 weeks after surgery and regulates flow,” according to the American Academy of Ophthalmology (AAO). “These devices have shown success in controlling intraocular pressure (IOP) in eyes with previously failed trabeculectomy and in eyes with insufficient conjunctiva because of scarring from prior surgical procedures or injuries. They also have demonstrated success in complicated glaucomas, such as uveitic glaucoma, neovascular glaucoma, and pediatric and developmental glaucomas, among others,” according to the AAO EyeWiki website.
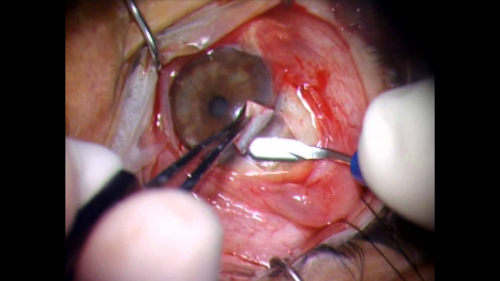
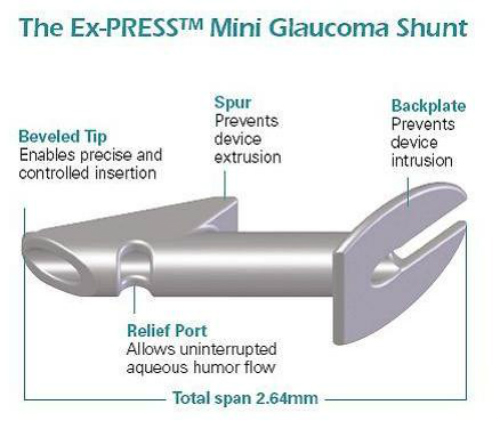
One such device, the ExPress mini glaucoma shunt (Figure 4) has been available internationally for nearly 20 years and has been implanted in nearly 100,000 patients worldwide. The device is designed to shunt aqueous from the anterior chamber to a subconjunctival reservoir. A trabeculectomy creates a conjunctival reservoir or filtering bleb.
(Image courtesy of Alcon/Novartis.)
The ExPress implant is a nonvalved stainless steel tube that was designed to improve upon standard trabeculectomy. (Figure 5.) The surgical technique does not require a sclerostomy or iridectomy. The device is inserted into the anterior chamber under a scleral flap. A prerequisite for patient eligibility for the ExPress implant is that there must be sufficient space within the anterior chamber angle for the device to be fitted. The ExPress is therefore contraindicated in acute and chronic angle closure, narrow angles, microphthalmia, and nanopthalmos.
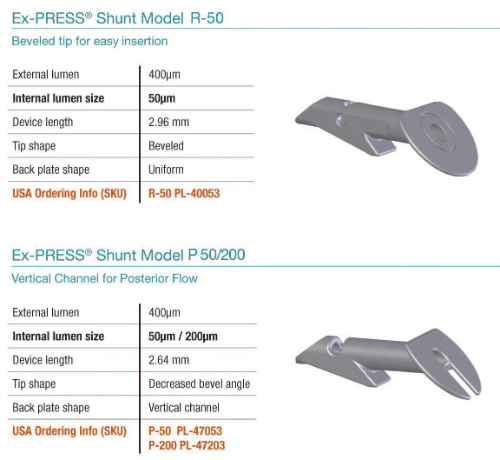
(Image courtesy of Alcon/Novartis.)
Following implantation of the Ex-Press device, aqueous is shunted to a subconjunctival reservoir, this occurs in a similar fashion as results from trabeculectomy, without removal of any sclera or iris tissue, according to a studies published recently in the International Journal Ophthalmology and the Journal of Ophthalmology.
The Ex-Press is manufactured by Alcon, a division of Novartis. The company website describes the device as follows:
“The EX-PRESS Glaucoma Filtration Device is intended to reduce IOP [intraocular pressure] in glaucoma patients when medication and conventional surgical treatments have failed. The device channels aqueous humor through a secure lumen (of either 50mcm or 200mcm) to a half-thickness scleral flap, creating a subconjunctival filtration bleb. This Lumenal Control means uniform filtration; uniform filtration helps to stabilize IOP during and after the procedure; and stable IOP means greater predictability.”
The Data
A systematic review and meta-analysis published recently in the International Journal of Ophthalmology found that both trabeculectomy and the ExPress device exhibited equivalent efficacy in reducing IOP. However, the ExPress was associated with a lower risk of hypotony and hyphema than was trabeculectomy.
This review included 11 studies that compared the efficacy of the ExPress versus trabeculectomy. The efficacy of ExPress was found to be similar to that of trabeculectomy with regard to reduction of IOP at 1-year and 2-year followup. (P=0.50 and weighted mean difference [WMD]: 2.89; 95% confidence interval [CI]: -8.05-13.83; P=0.60, respectively).
Implantation of the ExPress device was associated a significantly higher complete and qualified success rate (odds ratio [OR]: 1.59; 95% CI: 1.07-2.35; P=0.02 and OR: 1.74; 95% CI: 1.06-2.86; P=0.03, respectively). The frequency of hypotony and hyphema were significantly lower following the ExPress implantation procedure versus trabeculectomy (OR: 0.39; 95% CI: 0.21-0.72; P=0.003 and OR: 0.27; 95% CI: 0.10-0.69; P=0.003, respectively).
