Click here for the next report.
San Francisco—Patients with HCV genotype 3 have few effective therapies to date, particularly if they have cirrhosis. That may soon change, based on a pair of phase 3 trials. V Leroy, et al, reported on the ALLY-3+ Phase 3 Study at the 2015 meeting of the AASLD. Dr. Leroy presented Late Breaking abstract 3 (LB-3). Click here to find the entire abstract.
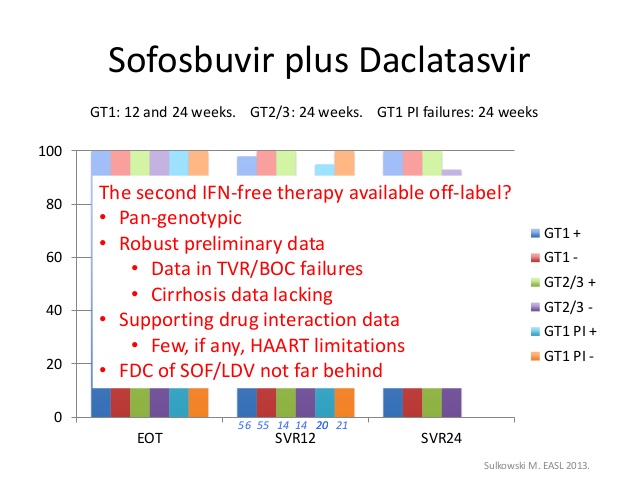
The previously reported ALLY-3 study found that patients who took 12 weeks of daclatasvir and sofosbuvir achieved a 96% sustained virologic response at post-treatment week 12 in the absence of cirrhosis and a 63% SVR12 with cirrhosis.
The ALLY-3+ study report indicates that adding ribavirin can boost results 50% in patients with cirrhosis and compensated advanced fibrosis. Patients with cirrhosis who took the 3-drug combination for 12 weeks achieved an 83% rate of sustained virologic response 4 (SVR4) weeks following treatment. Those who continued the therapy for 16 weeks saw a 94% SVR4. Patients with advanced fibrosis achieved an SVR4 of 100% following treatment of either 12 or 16 weeks.
The study included 50 patients; 24 treated for 12 weeks and 26 treated for 16. Most participants were white (80%) and male (98%); 72% had cirrhosis. Just over a quarter (26%) were treatment naïve and 10% had previously experienced treatment failure on a combination of sofosbuvir and ribavirin. Two patients relapsed in the 12-week arm and one in the 16-week arm. Of the 5 patients who had previously relapsed following treatment with sofosbuvir and ribavirin, 4 achieved SVR4.
One study participant died from non-treatment-related causes. No participants discontinued treatment due to adverse events. The most common adverse events were insomnia, fatigue and headache, all reported by 24% to 30% of participants.