Click here for the next article in the series.
Non-small cell lung cancer (NSCLC) is a complex disease that affects multiple aspects of a patient’s life. However, newer agents, multidisciplinary approaches, aggressive case management, and a focus on individual patients’ desires and goals of care can improve the experience of both patients and physicians.
With a combination of surgery, radiation, and now targeted agents, oncologists and patients have access to options that are more effective at improving survival while producing fewer negative side effects.
Currently, surgery with tissue sparing continues to be the first-line option in stages I and II NSCLC. For patients who are not ideal candidates for surgery, stereotactic radiation is considered an effective alternative. As the severity of NSCLC increases, oncologists may use platinum-based adjuvant regimens and chemoradiation.
Recent Advances
However, recent advances have opened the door for novel and targeted options in NSCLC – specifically, agents targeting anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR). Likewise, patients who have NSCLC who receive immunotherapeutic agents that target checkpoint pathways involving cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1), and PD-1 ligand (PD-L1) are showing clinical promise. (See Figure 1.) These novel agents have shown improve both efficacy and the patient’s experience and expectations regarding side effects.
Novel Agents
Agents Targeting Epidermal Growth Factor Receptor
The epidermal growth factor receptor (EGFR) is estimated to be overexpressed in 80% of NSCLC tumors, and the pathway involves tumor proliferation, invasion, and growth.
The most common toxicities associated with the EGFR class are diarrhea and rash. The rash is graded on a scale of severe to moderate to mild, with signs ranging from whole-body inflammatory involvement to skin fissures to dryness. Interventions to improve comfort include topical hydrocortisone, humidifiers, and sunscreen on sun-exposed areas. Oral antibiotics may provide anti-inflammatory benefits as well.
Gastrointestinal discomfort and upset are associated with EGFR inhibitors, and the dosing schedule may have to be adjusted to improve patient experience. Severe reactions may be managed by holding the dose or doses and by administering steroids for concomitant skin reactions involving more than 70% of the body surface area.
Anaplastic Lymphoma Kinase Gene Inhibitors
The ALK fusion oncogene is frequently involved in lung cancer in younger patients who have little to no smoking history and adenocarcinoma.
The anaplastic lymphoma kinase gene inhibitors (ALKGIs) have been shown to be effective in disease that progresses despite prior interventions. However, nearly 60% of patients require a dose reduction because of side effects, such as diarrhea, vomiting, and nausea. Likewise, monitoring of liver function is required because of risks for elevated alanine transaminase (ALT) and aspartate transaminase (AST) levels. More serious adverse reactions reported include interstitial lung disease, pneumonitis and pneumonia, and hypoglycemia.
Other ALKGIs have been associated with nausea, vomiting, pneumonitis, and abnormal liver function tests. However, they tend to be well tolerated. Early in the course, male patients may experience hypogonadism requiring testosterone monitoring and possibly replacement. Later in the course, lower extremity edema may develop. Finally, visual disturbances (the appearance of haloes and trailers) have been reported and are typically self-limited.
Angiogenesis Inhibitors
Serious toxicities have been reported in association with the intravenous angiogenesis inhibitors (See Figure 2.), including hemorrhage associated with the tumor, pulmonary hemorrhage, hypertension, and thrombosis.
Figure 2. The angiogenic process.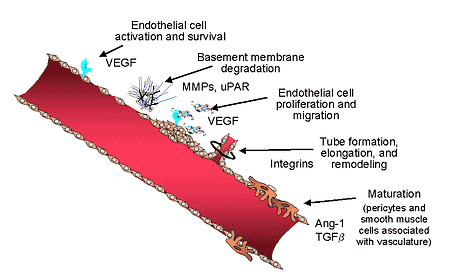
Other angiogenesis inhibitors work by inhibiting EGFR and vascular endothelial growth factor receptor 2 (VEGFR-2). The toxicities associated with these agents include nausea, diarrhea, hypertension, headaches, and rash.
Bleeding associated with anti-VEGF remains difficult to manage because this complication is partially intrinsic to the effectiveness. Likewise, the risk for bleeding is compounded by the risk for the formation of both venous and arterial thrombi.
Immunotherapies
Patients with hypertension related to angiogenesis inhibitors may require closer monitoring of their blood pressure and the initiation of antihypertensive agents.
Immune checkpoint inhibitors promote T-cell immune responses and therefore may lead to immune-mediated toxicities. (See Figure 3.) However, these modalities are still under investigation for lung cancer. Immunotherapies have been associated with initial increase in tumor volume, then subsequent shrinkage and delayed response, and these factors should be considered when the therapeutic effectiveness is assessed.Immunotherapies inhibit the pathways of PD-1 and CTLA-4, which are associated with the activation of tumor-specific T-cell antigens and possibly self-antigens. Dermal adverse events are the most common (12%–44%), followed by gastrointestinal effects (9%–32%), infusion reactions, adrenal insufficiency, hypothyroidism, and pneumonitis.
To prevent the recurrence of adverse events, slow tapering of glucocorticoids was used. For adverse events more severe than grade-2 pneumonitis, adrenal insufficiency, colitis, or diarrhea, hospitalization and specialist consultation were occasionally necessary. Once the adverse event had been managed, patients were usually restarted in clinical trials.
Immune-related toxicities tend to be low grade but may be swift and require prompt intervention. Adverse events may appear in the first cycle, after many cycles, or even after discontinuation. Strategies to mitigate toxicity during clinical trials include holding dose and administering glucocorticoids for gastrointestinal and hepatic events. Similarly, glucocorticoids and holding doses were used for early pneumonitis.
Phase II and III clinical trials are under way for vaccines in NSCLC. Interestingly, no adverse events have been reported in studies of these agents, both in the U.S. and in the United Kingdom. These agents initially appear to be well-tolerated and effective.
Further Reading
- American Cancer Society. Side effects of targeted cancer therapy drugs. Revised December 11, 2014. Accessed August 24, 2015.
- Bath C. Lung cancer expert offers recommendations for treating toxicities associated with targeted therapies. The ASCO Post. December 15, 2014. Accessed August 24, 2015.
- Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER), U.S. Food and Drug Administration (FDA). The voice of the patient: a series of reports from the U.S. Food and Drug Administration’s (FDA’s) patient-focused drug development initiative. Lung cancer. Published December 2013. Accessed August 24, 2015.
- Davies M. New modalities of cancer treatment for NSCLC: focus on immunotherapy. Cancer Manag Res. 2014;6:63-75.
- Elice F, Rodeghiero F. Side effects of anti-angiogenic drugs. Thromb Res. 2012;129 Suppl 1:S50-S53.
- Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382(9893):709-719.
- Silva S, Danson S. Targeted therapy and new anticancer drugs in advanced disease. Thorac Surg Clin. 2013;23(3):411-419.
- Simmons CP, Macleod N, Laird BJ. Clinical management of pain in advanced lung cancer. Clin Med Insights Oncol. 2012;6:331-346.
- Simone CB, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(4):178-188.
- Sundar R, Soong R, Cho BC, Brahmer JR, Soo RA. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer. 2014;85(2):101-109.
