Click here for the next article in the series.
As healthcare transitions from volume-based to value-based reimbursement, healthcare organizations are under pressure to ensure the delivery of efficient, effective, safe, and high-quality care while containing costs.
Increasingly, payers are looking to biomedical research to provide them with clinical knowledge that can drive these goals. Against this backdrop, the concept of performance-based medicine has arisen.
Lung cancer is a leading cause of mortality in the United States. More than 225,000 cases are diagnosed annually, 85% to 90% of which are of the non-small cell type.1 (See Figure 1.) An important contributor to the poor prognosis is the late stage at detection. Lung cancer is a major focus of recent developments in performance-based medicine from the perspective of healthcare providers and payers, as well as the lay public.
Although significant improvements in survival have been achieved for other forms of cancer, the survival rate for lung cancer is low and has remained largely unchanged for decades.2 However, recent technological advances have the potential to change the prognosis for patients with lung cancer through improved accuracy in diagnosis and personalized, specifically targeted agents.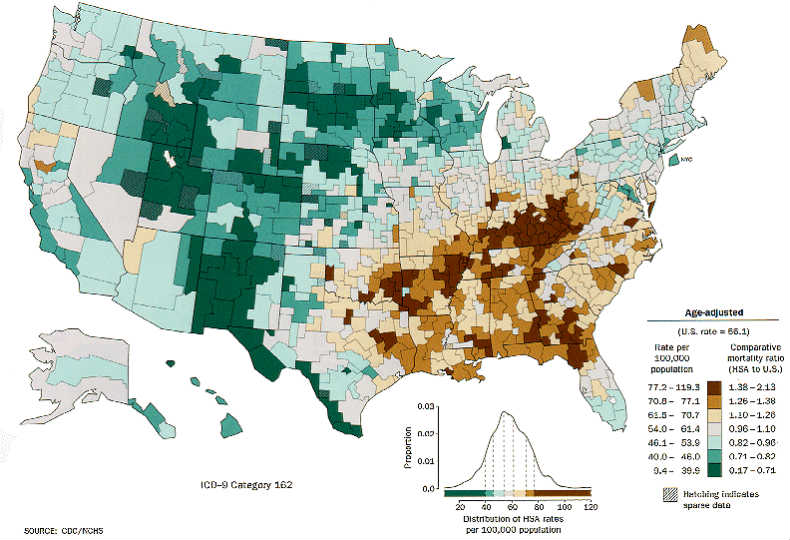
Diagnosis
The initial suspicion of lung cancer is most commonly preceded by abnormal findings on a chest radiograph or the appearance of local or systemic signs. The method of diagnosis of lung cancer depends on several factors, including cell type, tumor size and location, and the presence of metastasis, among others. The yields of sputum cytology and bronchoscopy – the primary conventional diagnostic tools – vary according to tumor location.
New tools have the potential to increase the accuracy and precision of diagnosis. Radial endobronchial ultrasound-guided lung biopsy and electromagnetic navigation bronchoscopy are emerging technologies with increased accuracy in the diagnosis of peripheral lesions.
Transthoracic needle aspiration and biopsy have been found to have sensitivity greater than that of bronchoscopic procedures. Pleural fluid cytology is useful for the diagnosis of a malignant pleural effusion, whereas pleural biopsy is diagnostic for metastatic disease involving the pleura, with thoracoscopic biopsy carrying the greatest accuracy. Evidence-based guidelines for the use of the various diagnostic tests have been published by Rivera et al.3
Performance-based medicine emphasizes the use of appropriate diagnostic methods and adequate tissue sampling to distinguish small cell from non-small cell tumors, and to improve outcomes. (See Figure 2.)
Screening
Technological advances in diagnosis have evolved to the point at which many lung cancers can be diagnosed before becoming clinically apparent. The National Lung Screening Trial evaluated screening in more than 50,000 high-risk individuals with low-dose computed tomography (LDCT). Despite a higher number of false-positive test results, the use of LDCT reduced lung cancer mortality by 20% and detected more cancers at earlier stages compared with chest radiography.4
In recognition that the early detection of preclinical lesions has the potential to improve outcomes, the Centers for Medicare & Medicaid Services proposed in November 2014 an annual “lung cancer screening counseling and shared decision making visit,” including LDCT, for asymptomatic individuals aged 55 to 77 years who have a tobacco smoking history of at least a 30 pack-years and currently smoke or have quit smoking within the past 15 years.5
An actuarial study of lung cancer screening in this high-risk population found that both the cost of screening and the cost per life-year saved were comparable with published rates for cervical, breast, and colorectal cancer.6 Effective screening heralds a paradigm shift in lung cancer that may convert the disease to one that is considered chronic.
Staging and Patient Selection
The accurate assessment of mediastinal disease is a critical part of determining whether a patient is a candidate for surgical resection. Conventional staging with computed tomography (CT) is limited by low sensitivity and specificity. Advances in positron emission tomography (PET) have increased the accuracy of mediastinal staging. In a recent meta-analysis, the median sensitivity and specificity of PET were 85% and 90%, respectively, compared with 61% and 79% for CT.7
Surgical decision making depends on accurate staging, and PET has been investigated as a way to improve the selection of patients for surgery.8 The combination of PET and CT may provide additional benefit in this regard.9
The conventional approach to tissue sampling in the mediastinum has been mediastinoscopy. Endobronchial ultrasound has emerged as a new tool to improve the accuracy of bronchoscopic needle biopsy, providing diagnostic effectiveness comparable with that of mediastinoscopy in a less invasive manner.10 As described by Pillai and Ramalingam, “[t]he use of PET and EBUS has revolutionized the management of early-stage lung cancer and improved surgical outcomes by optimizing patient selection.”11
Pathology Plus Molecular Biology Equals Personalized Medicine
Personalized therapy in cancer requires an oncogenic target, a predictive biomarker, and clinical evidence documenting treatment efficacy.12 The surgical pathologist’s traditional emphasis on the differentiation of small cell from non-small cell lung cancer (NSCLC) types has evolved to include detailed histologic and molecular classification.2
Advances in genomics have led to the identification of several unique mutations in lung cancer that support the goals of performance-based medicine optimizing outcomes. Several therapeutic agents have been developed targeting tumor-specific histologic features in NSCLC, including vascular endothelial growth factor (VEGF), folate metabolism, purine and pyrimidine synthesis, and the EML4-ALK tyrosine kinase translocation.
The Lung Cancer Mutation Consortium (LCMC), a collaborative approach involving 16 academic centers, is “actively promoting molecular mutation testing in lung cancer in order to match patients to optimal treatment strategies.”13 Initial reports in 2011 included 10 driver mutations in 516 patients with advanced adenocarcinoma, 97% of which were mutually exclusive. Subsequent follow-up of more than 1000 patients revealed that 28% of the patients had been directed to a targeted therapy for their mutation. The median survival of patients with a driver mutation who received a targeted therapy was 3.5 years, compared with 2.4 years in those who did not receive a targeted therapy.14
The National Cancer Institute in August 2014 launched 3 integrated precision medicine trials – the Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials (ALCHEMIST) – to identify early-stage tumors with specific genetic characteristics and evaluate the outcomes of targeted agents, including survival.15 Continued research to identify new driver mutations has the potential to change the landscape of treatment for NSCLC.11
Future Directions
Advances in molecular testing in lung cancer have created several technological challenges, such as ensuring the adequacy of sampling with minimally invasive biopsy techniques and evaluating tumors for genetic heterogeneity. Potentially greater challenges include distinguishing the relative importance of multiple genetic mutations and decision making in the face of incomplete information regarding efficacy. There may be uncertainty regarding the benefits and harms of personal genomic information,16 and cost-benefit analyses will likely lag behind the development of new genetic testing platforms.14 Teams of diverse types of specialists will be needed to gather and interpret genomic information in such a way that research findings can be incorporated effectively into clinical practice.
References
- American Cancer Society. What are the key statistics about lung cancer. Revised March 4, 2015. Accessed August 23, 2015.
- Cagle PT, Myers J. Precision medicine for lung cancer: role of the surgical pathologist. Arch Pathol Lab Med. 2012;136: 1186-1189. Accessed August 23, 2015.
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S-e165S. Accessed August 23, 2015.
- National Lung Screening Trial Research Team (NLSTRT). Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
- Centers for Medicare & Medicaid Services. Decision memo for screening for lung cancer with low-dose computed tomography (LDCT) (CAG00439-N). Published February 5, 2015. Accessed August 23, 2015.
- Pyenson BS, Sander MS, Jiang Y, Kahn H, Mulshine JL. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff. 2012;31(4), 770-779. Accessed August 24, 2015.
- Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139(11): 879-892.
- van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359(9315):1388-1393.
- Lardinois D, Weder W, Hany TF, et al. Staging of non-small cell lung cancer with integrated positron emission tomography and computed tomography. N Engl J Med. 2003;348(25): 2500-2507.
- Herth FJ, Eberhardt R, Vilmann P, Krasnik M, Ernst A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61(9): 795-798.
- Pillai RN, Ramalingam SS. Advances in the diagnosis and treatment of non–small cell lung cancer. Mol Cancer Ther. 2014;13(3):557-564.
- Mok TS. Personalized medicine in lung cancer: what we need to know. Nat Rev Clin Oncol. 2011;8(11): 661-668.
- Shames DS, Wistuba II. The evolving genomic classification of lung cancer. J Pathol. 2014;232:121-133. Accessed August 24, 2015.
- Johnson BE, Kris MG, Berry LD, et al. A multicenter effort to identify driver mutations and employ targeted therapy in patients with lung adenocarcinomas: The Lung Cancer Mutation Consortium. Presented at: Poster Discussion Session, 2013 ASCO Annual Meeting. J Clin Oncol. 2013;31(suppl; abstr 8019). Accessed August 24, 2015.
- National Cancer Institute. NIH announces the launch of 3 integrated precision medicine trials; ALCHEMIST is for patients with certain types of early-stage lung cancer. Posted August 18, 2014. Accessed August 23, 2015.
- Ramsey SD, Veenstra D, Tunis SR, Garrison L, Crowley JJ, Baker LH. How comparative effectiveness research can help advance ‘personalized medicine’ in cancer treatment. Health Aff. 2011;30(12): 2259-2268. Accessed August 23, 2015.
